Introduction
The inhibitory and lethal effects of ozone on pathogenic organisms including viruses have been observed since the latter part of the 19’th century. (Shentag, Akers, Ph.D., Campagna, & Chirayath)
Viruses are very small microscopic particles containing DNA or RNA, lacking the capacity to reproduce themselves outside of a host. Viruses are smaller than bacteria or fungi. They attach themselves to living cells injecting their genetic code into the host, taking over the host’s cellular machinery to reproduce themselves at a rapid rate.
Covid-19, otherwise known as SARS-CoV2 is an enveloped coronavirus, which means that the viral genetic material exists inside a lipid envelope within a protein capsule. The structure of Covid-19 is very similar to that of the Severe Acute Respiratory Syndrome (SARS-CoV1) virus, which is also an enveloped corona virus, sharing over 80% of the same genetic code. The Middle East Respiratory Syndrome (MERS-CoV) virus is also a coronavirus.
Table 1 shows the relative ease of disinfection (biocide susceptibility) for various pathogens, reproduced from The Handbook of Water and Wastewater Microbiology. (Microbial Response to Disinfectants, 2003)
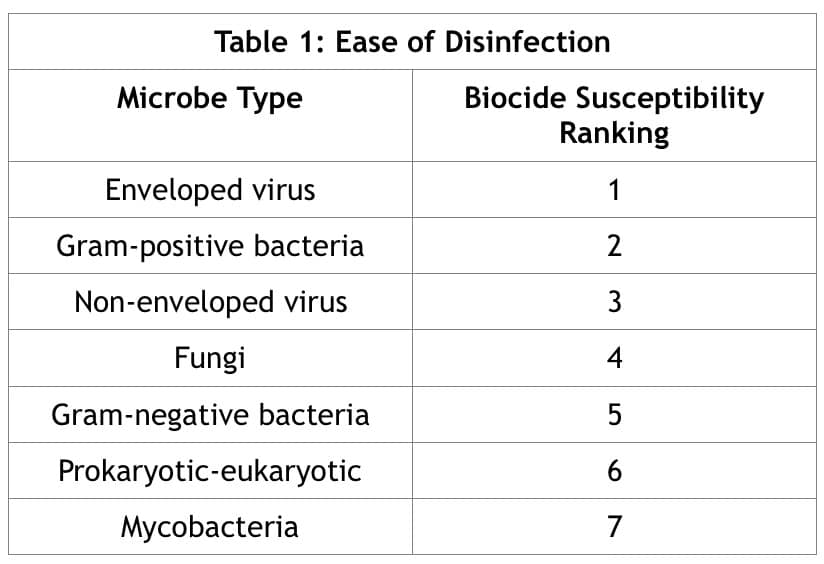
In the United States, in order to make claims regarding the performance of a disinfectant such as ozone, the product must be listed by the EPA. The process allows for rapid approval in “Section V, Outbreak criteria associated with Emerging Pathogens”, diseases that appear in the human population for the first time. Application requires classification of the virus into large and small non-enveloped viruses, and enveloped viruses. Instructions state “Enveloped viruses are the least resistant to activation by disinfection”, and lists the viral families accepted in this category showing Coronaviridae, which includes COVID-19. These EPA instructions are consistent with claims by others regarding the ease of disinfecting coronaviruses. (EPA, 2016). We recently applied for registration as a disinfectant for COVID-19 based on the data in this report.
Breathing ozone gas can be hazardous to human health. The Occupational Safety and Health Administration (OSHA) has set Public Health Air Standards of 0.1 ppm for 8 hours or 0.3 ppm for 15 minutes as the limit of the amount of ozone to which people can be safely exposed. Air cleaners based on ozone should not generate ozone levels above the Public Health Standards, which are considered below any disinfectant activity. (Shentag, Akers, Ph.D., Campagna, & Chirayath)
Studies of Ozone use for Decontamination of Viruses
Zhang, et al, (Zhang, Ziao, Zhou, & Gao, 2004) demonstrated that SARS-CoV1 could be destroyed by 27 ppm of ozone in solution applied for four minutes. SARS-CoV1 is nearly identical in structure to COVID-19. Ozone solutions at concentrations of 18 ppm, and 5ppm were also effective.
Hudson, et.al. (Hudson, Sharma, & Vimalanathan, 2009) dried 12 different virus strains onto different surfaces that were placed into a test office for ozone treatment with low (38%) and high (70%) relative humidity. The selected viruses were known pathogens, including influenza, and mouse coronavirus, which are both enveloped viruses, like COVID-19. The ozone generator delivered up to 20 ppm for 20 minutes. Reduction in viral count depended strongly on relative humidity, with an average of 32% reduction at low humidity, and an average reduction of 99.8% at high humidity.
The Hudson study also evaluated the ability of ozone to mitigate viral aerosols. A liquid suspension of Influenza virus was sprayed into a laboratory chamber with and without 20 ppm ozone, and viable viral counts were measured in the condensate. On average, 99.73% of the virus were inactivated from the aerosol, indicating that ozone also deactivates aerosol born viruses.
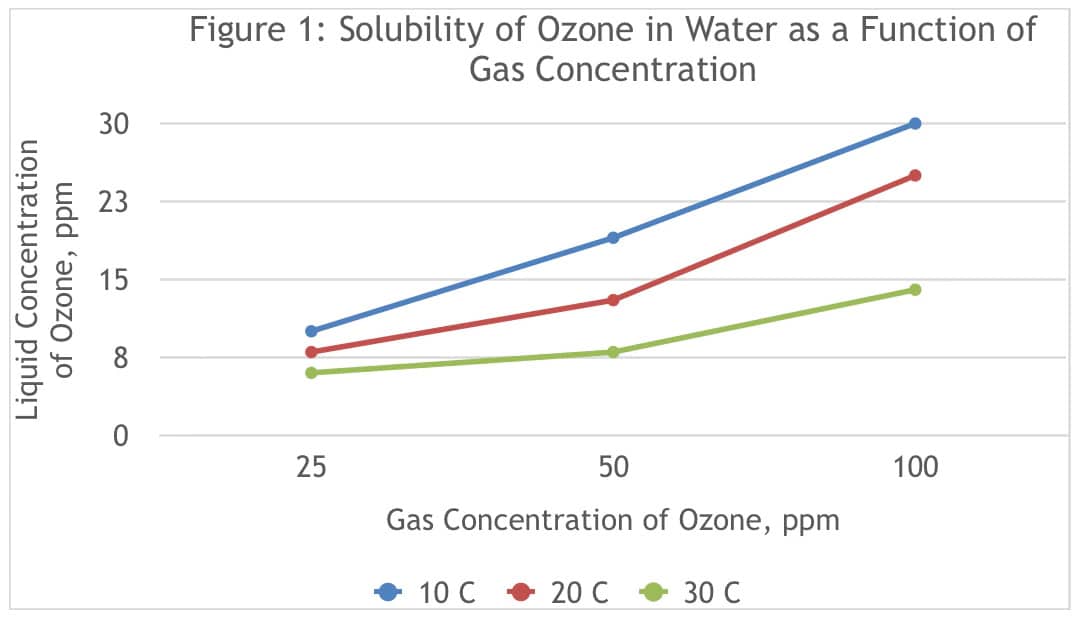
In order for ozone to deactivate viruses in aerosols, ozone must be sufficiently solubilized in water, so that the ozone can reach the virus. Figure 1 shows the solubility of ozone at different gas-phase concentrations of ozone, and at various temperatures. (Ozone Solutions, n.d.)
On average these results show that the ozone concentration solubilized in water is 26% of the gas-phase concentration at 20 degrees Celsius, indicating that ozone can penetrate into aerosol droplets at meaningful concentrations. This figure also shows how the solubility of ozone in water increases with decreasing temperature.
Tseng and Li (Tseng & Chihshan, Volume 70. No. 19 (June 2008)) measured the survival curves of four different viruses on a Gelatin-based medium over a range of ozone concentrations (600 ppb, 900 ppb, and 1200 ppb) at 55 and 85% relative humidity, for a time period of up to 120 minutes. Table 2 shows selected results from the study.
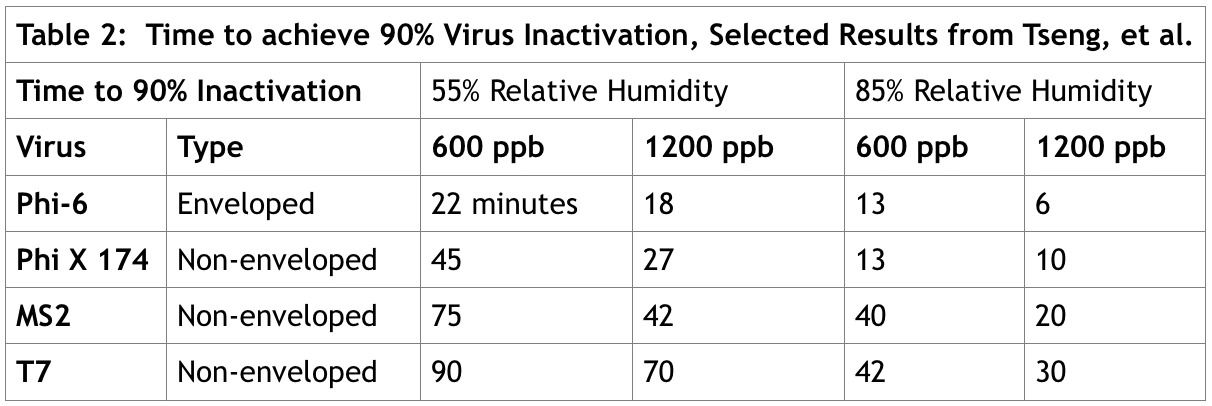
The results show that the enveloped virus Phi-6 required the least amount of time for deactivation across all ranges of ozone concentration and relative humidity. For all viruses, increasing ozone concentration significantly reduced the time required for deactivation. Increasing relative humidity also had a significant impact on reducing the time to deactivate the viruses.
Roy & Wong (Roy & Wong, Mechanism of Enteroviral Inactivation by Ozone, 1981) studied how ozone treatment impacts the viability of poliovirus. Poliovirus is a non-enveloped virus that is transmitted into its host through the gut. Treating the system with 0.5 mg/liter (500 ppb, or 0.5 ppm) of ozone for ten seconds destroyed over 90% of the virus. This study also discovered that ozone damaged the protein coat of the virus, although this damage did not appear to impact the viability of the virus. Instead, the ozone directly attacked the viral RNA, and this mechanism was attributed to the reduction in survival.
Roy, Chain, (Roy, Chian, & Engelbrecht, Kinetics of Enteroviral Activation of Ozone, 1981) et al, shown that Ozone’s viricidal effect is attributed to damaging the viral RNA, through oxidation of the viral nucleic acid (RNA). The rate of viral disruption is determined by the mass transfer rate of the ozone into the viral molecules.
There is a widely found quote on the internet claiming that ozone gas has been proven to destroy surface-born SARS coronavirus. “There are more than seventeen scientific studies that show Ozone gas is capable of destroying the SARS coronavirus” (Thailand Medical news, 2020). However, these seventeen references were not provided in the document.
Modeling of Virus Destruction
A regression model of the time required to deactivate a virus using ozone and humidity was developed based the Tseng et al. data discussed above. This model was designed to provide accurate interpolation and extrapolation of the data set using the specific ozone concentrations, humidity, and time exposures encountered in field applications. Data for virus Phi X 176, which is a non-enveloped virus, were used in the regressions. This Phi X 176 basis was chosen to provide a significant measure of conservancy in the estimates (Table 2), given the enveloped Phi-6 virus is most similar to COVID-19 and much easier to deactivate.
The resultant model is:
NS/N0 = exp(-K*CT)
NS = Concentration of surface virus surviving after exposure to ozone, PFU/mL
N0 = Concentration of surface virus unexposed to ozone, PFU/ml
CT = Contact time, minutes
K = Virus susceptibility factor
The regression model for K is as follows:
K = 1.437E-7 * (ppb)^2 + 0.093147 * RH
ppb = parts per billion ozone in the gas phase
RH = relative humidity, percent
The t-statistic for the coefficients in the K model was 7.4 for the ppb term, and 2.5 for the relative humidity term, indicating that these parameters are statistically significant above the 95% confidence interval. The overall R-squared for this model is 0.993, which is exceptional.
Figure 3 is a graphical representation of the required deactivation time to achieve 99 percent removal of the virus based on this model.
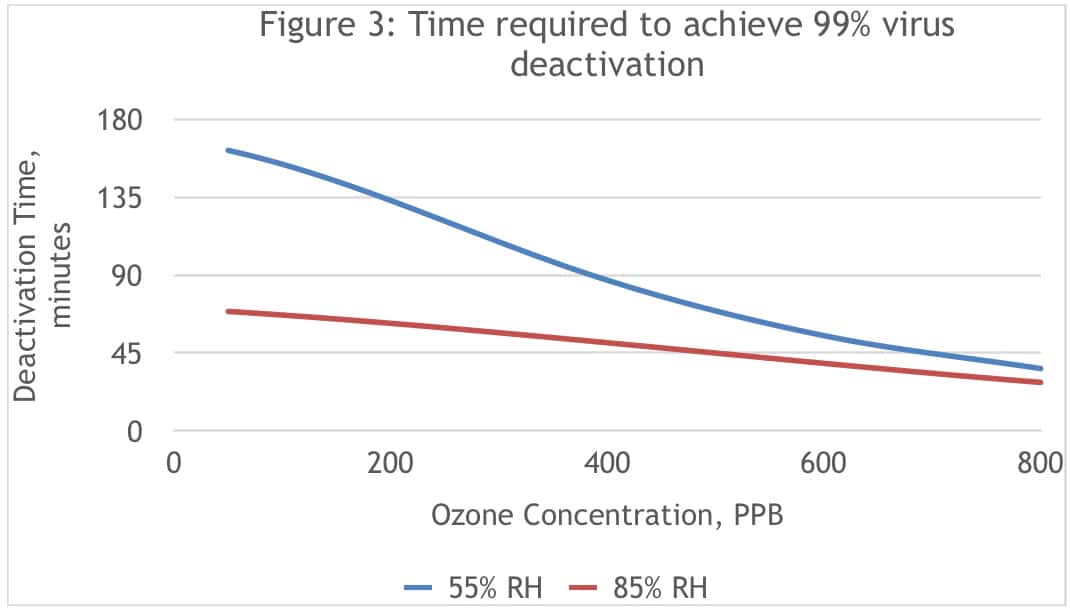
The model shows how increasing ozone concentration reduces deactivation time, and that deactivation occurs faster at high humidity levels, particularly at lower ozone concentrations.
Discussion
Ozone at low concentrations in the parts per billion level can be used to deactivate viruses such as COVID-19. Adding humidity can be used to accelerate the deactivation of viruses at low ozone concentrations.
Ozone has the advantage over other disinfectants that it is a gas, so that it can permeate all areas of a room, and deactivate the hardest to reach areas, including the ventilation system. Ozone has the additional advantage that it can disinfect aerosols suspended in the air. Additionally, ozone decomposes to oxygen so it is residue free.
Please feel free to contact me if you have any questions?
Rick Wilson, Ph.D.
[email protected]
(650) 704-8477
Bibliography
- Doremalen, N., Morris, D. H., Holbrook, M., & Gamble, A. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. The New England Journal of Medicine, Correspondence.
- Elvis, A., & Ekta, J. (2011, Jan-June). Ozone Therapy: A clinical review. Journal of the Natural Science Biological Medicine, 66-70.
- EPA. (2016, August 19). Pesticide Registration. Retrieved from EPA United States Environmental Protection Agency: https://www.epa.gov/pesticide-registration/guidance-registrants-process-making-claims-against-emerging-viral-pathogens
- Hudson, J. B., Sharma, M., & Vimalanathan, S. (2009). Development of a Practical Method for Using Ozone Gas as a Virus Decontamination Agent. Ozone: Science and Engineering, 31, 216-223.
- Lam, K. (n.d.). Ozone Disinfection of SARS-Contaminated Areas. Retrieved from Ozone Tech: https://www.ozonetech.com/sites/default/files2/pdf/Ozone_disinfection_of_SARS_Contaminated_Areas.pdf
- Microbial Response to Disinfectants. (2003). In D. Mara, & N. Horan, The Handbook of Water and Wastewater Microbiology (Vols. 666, Chapter 39, page 561-618). San Diego, California: Elsevier Science.
- Muzhi, Z. (2020, February 26). Ozone: A powerful weapon to combat COVID-19. Retrieved from China.org.cn: http://www.china.org.cn/opinion/2020-02/26/content_75747237_4.htm
- Ozone Solutions. (n.d.). Ozone Solubility. Retrieved March 2020, from Ozone Solutions: https://www.ozonesolutions.com/knowledge-center/ozone-solubility.html
- Rabenau, H. F., Kampf, G., Cinatl, J., & Doerr, H. W. (2005). Efficacy of various disinfectants against SARS coronavirus. Journal of Hospital Infection, 107-111.
- Rowen, R. J., & Robins, H. (2020). A Plausible “Penny” Costing Effective Treatment for Corona Virus – Ozone Therapy. Journal of Infectious Diseases and Epidemiology, 6(2).
- Roy, D., & Wong, P. (1981). Mechanism of Enteroviral Inactivation by Ozone. Applied Enviornmental Biology, 41, 718-723.
- Roy, D., Chian, E., & Engelbrecht, R. (1981). Kinetics of Enteroviral Activation of Ozone. J. Environ.Eng. Div Am. Soc. Chem. Eng., 107, 887-901.
- Roy, D., Engelbrecht, R., & Chian, E. (1982, December). Comparative Inactivation of Six Enteroviruses by Ozone. Journal of the American Water Works Association, 660-664.
- Roy, D., Engelbrecht, R., Wong, P., & Chian, E. (1981). Inactivation of Enteroviruses by Ozone. Water Science Technology, 13, 819-836.
- Shentag, J. J., Akers, Ph.D., C., Campagna, P., & Chirayath, P. (n.d.). SARS: Clearing the Air. Retrieved from NCBI Bookshelf: www.ncbi.nlm.nih.gov/gooks/NBK92445
- Sunnen, MD, G. V. (2003, May). SARS and Ozone Therapy: Theoretical Considerations. Retrieved from https://www.ziondaily.com/2.0/web/health_10e/view.php?id=9389
- Thailand Medical news. (2020, February 5). Ozone Can Be Used to Destroy the New Coronavirus and Disinfect ARes. Retrieved March 2020, from Thailand Medical News: https://www.thailandmedical.news/news/ozone-can-be-used-to-destroy-the-new-coronavirus-and-disinfect-areas
- Tseng, C.-C., & Chihshan, L. (Volume 70. No. 19 (June 2008)). Inactivation of Surface Viruses by Gaseous Ozone. Journal of Environmental Health, 56-63.
- Zhang, J.-m., Ziao, G.-f., Zhou, Y.-q., & Gao, R. (2004). Examination of the Efficacy of Ozone Solution Disinfectant in an Activated SARS Virus. Chinese Journal of Disinfection.